Serial Dilution Sources Of Error In Measurement Physics
Dilutions: Dilutions can sometimes be visually observed. In the image above, the intense red color slowly fades as the solutions become more diluted.
Knigu irka hortica vibor vedjmi. 1998 Mar;54(1):19-32. The effect of serial dilution error on calibration inference in immunoassay. Higgins KM(1), Davidian M, Chew G, Burge H.
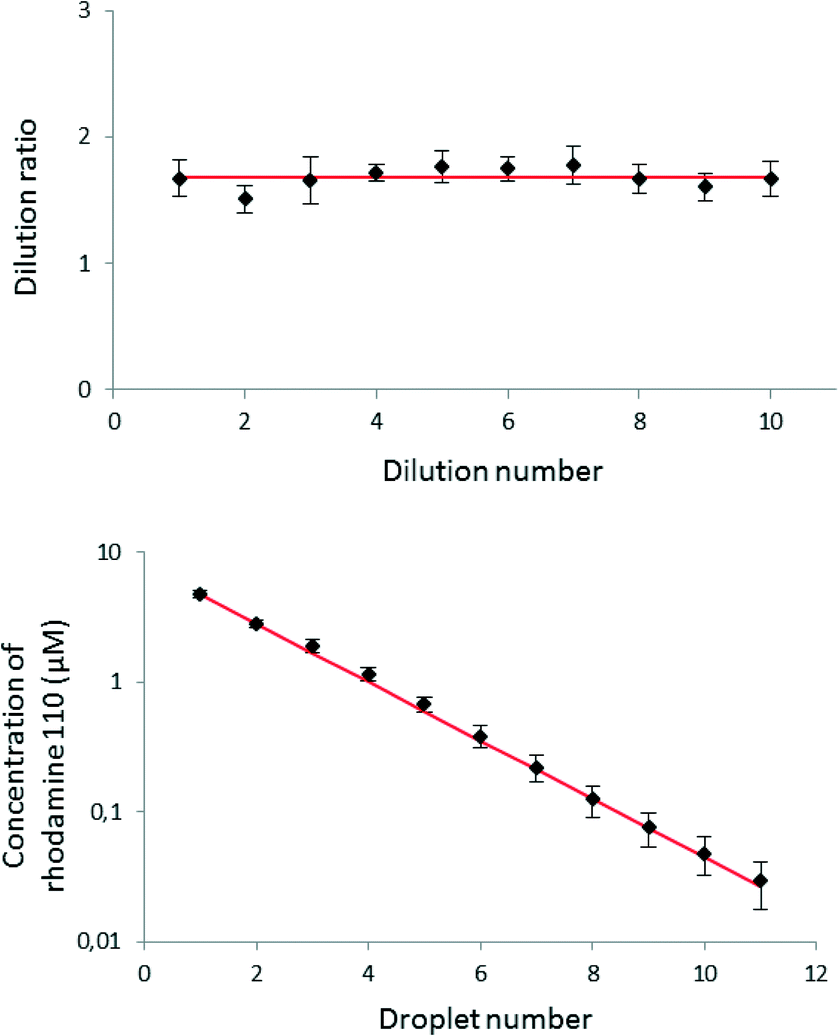
Serial Dilutions Serial dilutions involve diluting a stock or standard solution multiple times in a row. Typically, the dilution factor remains constant for each dilution, resulting in an exponential decrease in concentration. For example, a ten-fold serial dilution could result in the following concentrations: 1 M, 0.1 M, 0.01 M, 0.001 M, and so on. As is evidenced in this example, the concentration is reduced by a factor of ten in each step. Serial dilutions are used to accurately create extremely diluted solutions, as well as solutions for experiments that require a concentration curve with an exponential or logarithmic scale. Serial dilutions are widely used in experimental sciences, including biochemistry, pharmacology, microbiology, and physics.
Key Takeaways Key Points • Molar concentration, also called molarity, is the number of moles of solute per liter of solution. Molarity is the most common measurement of solution concentration. Berserk golden age arc 1 english dubbed. • Because molarity measurements are mole/L measurements, we often use this unit for stoichiometric calculations to determine the amount of chemical in a given mixture. • Do not confuse moles with molarity: molarity is a measure of concentration, while moles are a measure of the amount of substance. Key Terms • molarity: The concentration of a substance in solution, expressed as the number moles of solute per liter of solution.
• solution: a homogeneous mixture, either liquid, gas, or solid, formed by dissolving one or more substances • mole: Molarity In chemistry, molar concentration, or molarity, is defined as moles of solute per total liters of solution. This is an important distinction; the volume in the definition of molarity refers to the volume of the solution, and not the volume of the solvent.
The reason for this is because one liter of solution usually contains either slightly more or slightly less than 1 liter of solvent, due to the presence of the solute. The SI unit for molarity is is mol/m 3; however, you will almost always encounter molarity with the units of mol/L. A solution of concentration 1 mol/L is also denoted as “1 molar” (1 M). Mol/L can also be written in the following ways (however, mol/L, or simply M, is most common): 1 mol/L = 1 M = 1 mol/dm 3 = 1 mol dm −3 = 1000 mol/m 3 It is important to distinguish moles from molarity; molarity is a measurement of concentration while moles are a measure of the amount of substance present at a given time.
CC licensed content, Specific attribution • Sunil Kumar Singh, Dilution. September 17, 2013. Provided by: OpenStax CNX. License: • Serial dilution. Provided by: Wikipedia. License: • Sunil Kumar Singh, Dilution.